Respiratory Syncytial Virus
(RSV) Vaccine
Respiratory syncytial virus, or RSV, typically causes mild, cold-like symptoms, but it can lead to severe illness in some people. Children under age 5, and particularly babies 6 months and younger, are most vulnerable to severe RSV illness, even if they are healthy. Each year, thousands of young children are hospitalized because of RSV illness.
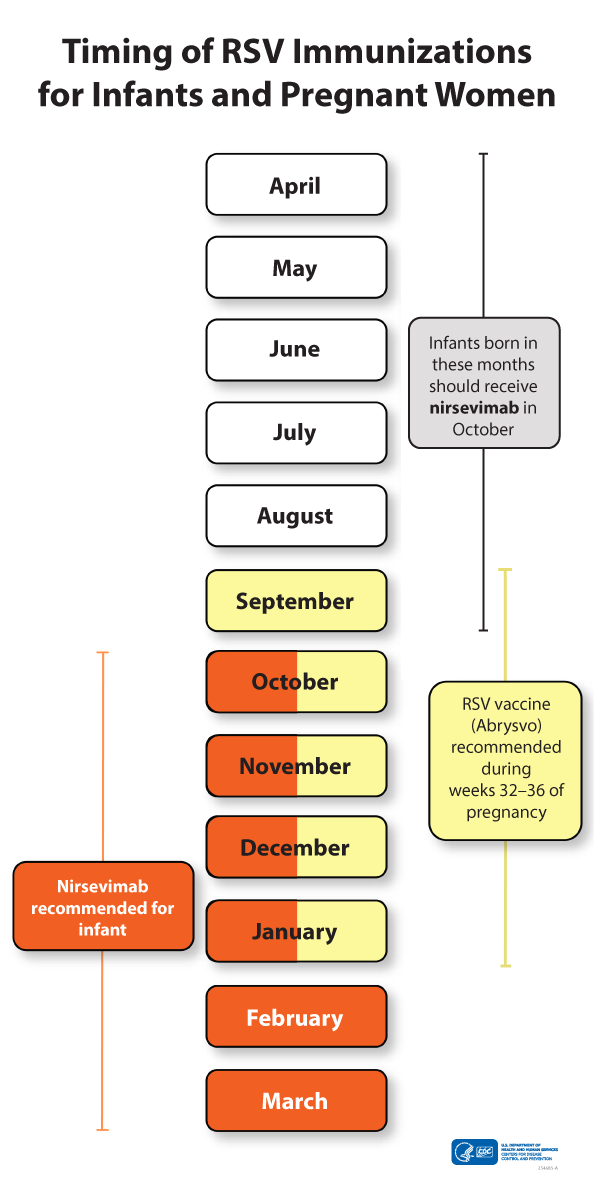
There are two ways to protect babies against RSV:
Pregnant women can get the RSV vaccine (Abrysvo) from 32 through 36 weeks of pregnancy during RSV season (September 1st to January 31st in most areas of the United States),
OR
Babies born during October through March can receive a single dose of an RSV-targeted monoclonal antibody, either nirsevimab (Beyfortus) or clesrovimab (Enflonsia). Either medication is ideally given at birth while the baby is still in the hospital, but they can be given after the baby goes home, up to 8 months of age. In addition, babies born from April through September, before their mothers could get the RSV vaccine, can get either nirsevimab or clesrovimab in October, at the start of RSV season.
Most infants do not need both maternal vaccination and monoclonal antibody.
-
Abrysvo is an RSV vaccine approved for use in pregnant women and older adults. It is given in pregnancy as a single dose from 32 through 36 weeks of pregnancy, during RSV season.
There is another RSV vaccine called Arexvy that is given to older adults. This vaccine is not approved for pregnant women. Only the Abrysvo vaccine has been FDA-approved for use in pregnancy.
-
The vaccine works with the body’s immune system to make antibodies against RSV. When the vaccine is given during pregnancy, these antibodies cross the placenta and enter the fetal bloodstream. This process takes about 2 weeks. When a pregnant woman receives the RSV vaccine, her baby will be born with enough antibodies to protect against RSV for at least the first 6 months of life.
-
Studies show that the RSV vaccine, when given during pregnancy:
Reduces the risk of serious illness from RSV infection in babies by 80% in the first 3 months after birth, with protection lasting at least 6 months.
Reduces the risk of a baby being hospitalized due to RSV by 67% during the first 6 months after birth.
-
The most commonly reported side effects are pain at the injection site, headache, muscle pain, and nausea.
-
A monoclonal antibody is made in a lab. It works like the body’s own antibodies to fight off certain diseases. Two antibody medications are available: nirsevimab (also called Beyfortus) and clesrovimab (Enflonsia). Both are given to babies after birth to protect against RSV infection and its severe complications. They both deliver anti-RSV antibodies directly to the baby. They are given to babies younger than 8 months old who are either born during RSV season or entering their first RSV season. Newborns can get nirsevimab or clesrovimab before they leave the hospital. A single dose of either medication has been shown to reduce the risk of severe RSV illness in babies for at least 5 months after being given.
-
Minor side effects include redness and swelling at the injection site and a mild rash. No serious side effects have been reported.
-
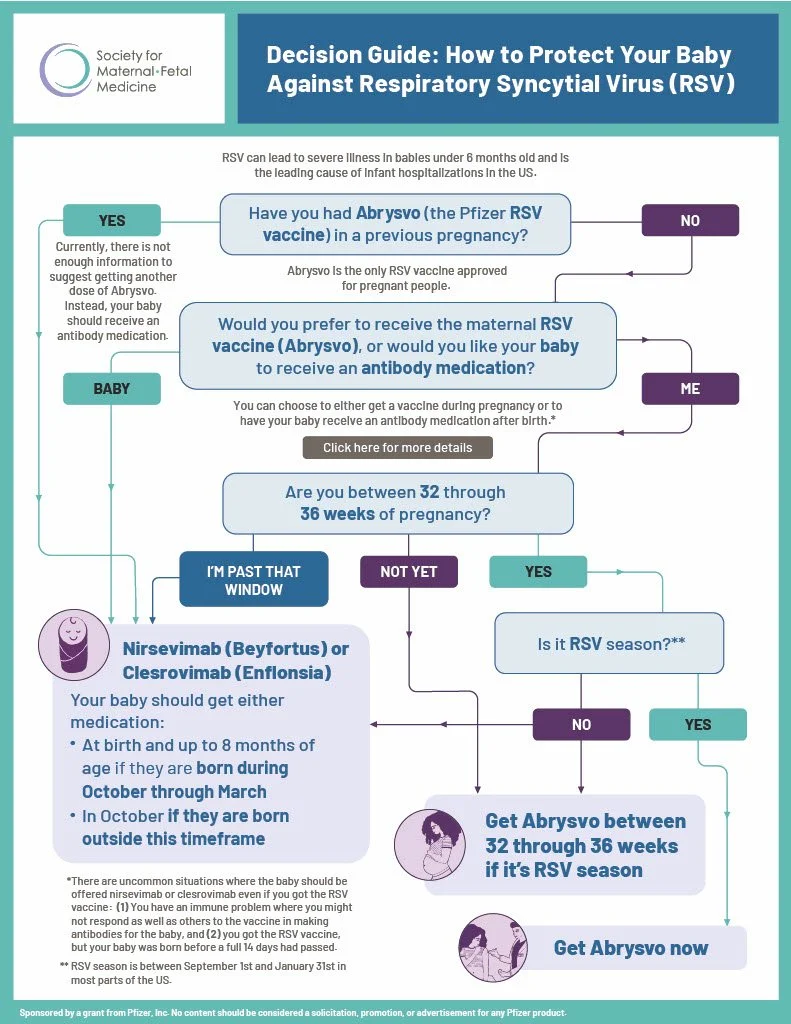
It depends. Women who received the Abrysvo vaccine during a previous pregnancy and are pregnant again should not get another Abrysvo vaccination. Instead, their babies should receive nirsevimab or clesrovimab if they are born during RSV season.
Women who have never gotten Abrysvo before can get Abrysvo from 32 through 36 weeks of pregnancy during RSV season, OR their baby can receive nirsevimab or clesrovimab from birth up to age 8 months.
The Abrysvo RSV vaccine given during pregnancy and the infant antibody medications nirsevimab (Beyfortus) and clesrovimab (Enflonsia) are both safe and effective ways to prevent illness caused by RSV in infants. Here is a comparison of the two options:
| ABRYSVO (RSV vaccine given during pregnancy) | NIRSEVIMAB (Beyfortus) CLESROVIMAB (Enflonsia) (RSV immunization given to babies) | |
|---|---|---|
| Who is it for? | Pregnant women who have not received Abrysvo in a previous pregnancy Given from 32 through 36 weeks of pregnancy during RSV season (September 1st through January 31st) | • Infants up to age 8 months entering their first RSV season, AND • Infants and children aged 8 – 19 months entering their second RSV season |
| How effective is it at preventing severe RSV in babies? | Reduces severe RSV in babies by 70-80% for at least 6 months after birth Reduces the risk of babies being hospitalized due to RSV by 50% for at least 6 months after birth | Reduces severe RSV in babies by 70-80% for at least 5 months after birth Reduces the risk of babies being hospitalized due to RSV by 80-90% within 5 months of birth |
| What are the side effects? | Injection site pain, headache, muscle pain, nausea, and fever | Rash and injection site pain |
| What are the benefits? | • The baby is born protected against RSV, because antibodies were passed from mother to baby before delivery. • Abrysvo may give better protection against RSV variants than nirsevimab. • It reduces the number of injections a baby gets. • There may be ongoing protection through breastfeeding. | The protection from nirsevimab or clesrovimab may be more long-lasting than with Abrysvo. |
Decision Guide: How to Protect Your Baby Against Respiratory Syncytial Virus (RSV) During Pregnancy
RSV Vaccine Safety and Timing
SMFM Vaccine Safety
SMFM Vaccine Timing
Maternal RSV Vaccine: What Patients Need to Know
Quick Facts
RSV can cause serious illness in infants, especially those under age 6 months, even in those who are healthy. Vaccination against RSV during pregnancy or giving the newborn an RSV-specific antibody medication can help protect babies from serious illness.
There are two ways to protect babies from RSV:
A vaccine (Abrysvo) given to the mother during pregnancy, or
An injection (nirsevimab or clesrovimab) given to the infant
The RSV vaccine Abrysvo is recommended for pregnant women from 32 through 36 weeks of pregnancy during RSV season, which typically lasts from September 1st to January 31st in the United States.
Abrysvo works with the body’s immune system to create RSV antibodies that pass through the placenta, providing babies with protection against RSV.
Abrysvo reduces the risk of severe RSV complications by 70% and hospitalization by 67% within six months of birth.
Another way to prevent RSV in babies is to give a monoclonal antibody, either nirsevimab or clesrovimab, after delivery. This medication is also highly effective in preventing severe RSV illness in newborns and infants.
Glossary
Antibodies: Proteins made by the immune system in response to a foreign substance, such as a virus.
Immune system: The cells and organs that protect the body against foreign substances, such as bacteria and viruses.
Monoclonal antibody: A lab-made antibody that can be given to help prevent or reduce the severity of certain diseases.
Placenta: A special organ that develops during pregnancy. It allows the transfer of nutrients, antibodies, and oxygen to the fetus. It also makes hormones that sustain the pregnancy.
This resource was supported by the Society for Maternal-Fetal Medicine (SMFM) and the Centers for Disease Control and Prevention (CDC) cooperative agreement CDC-RFA-DD-23-0004 Enhancing Partnerships to Address Birth Defects, Infant Disorders and Related Conditions, and the Health of Pregnant and Postpartum People. The views expressed by the authors do not necessarily reflect the official policies of the Department of Health and Human Services nor represent an endorsement by the U.S. Government.
The videos and decision tree on this page are sponsored by a grant from Pfizer, Inc. No content should be considered a solicitation, promotion, or advertisement for any Pfizer product.
Last Updated: August 2025